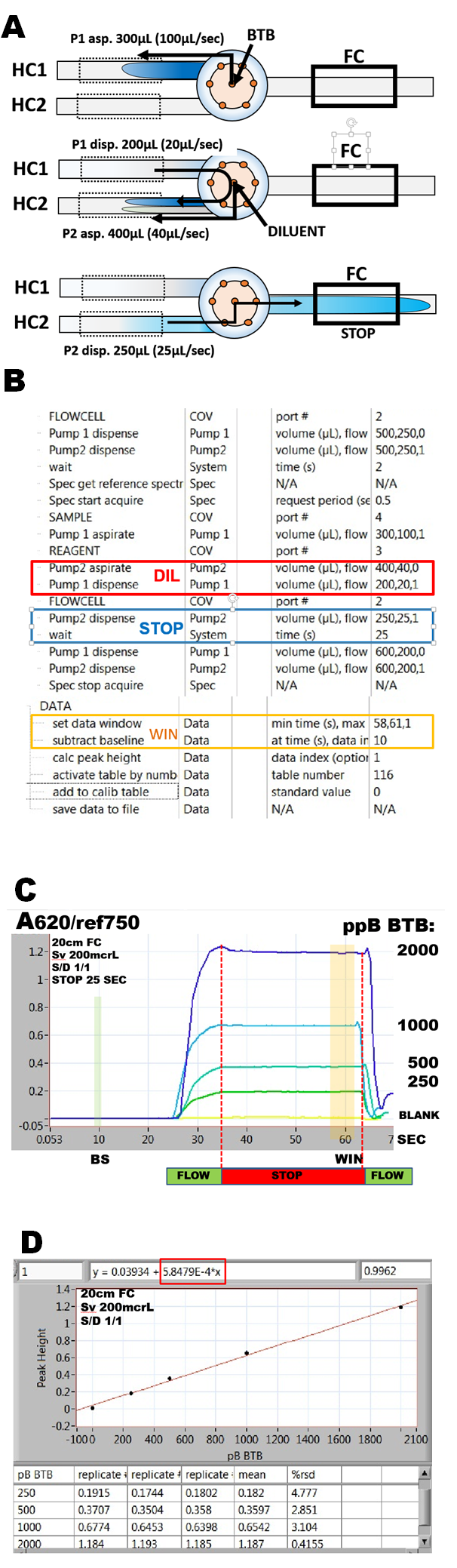
The flow protocol (A, B) comprises the following steps:
1) sample of BTB is aspirated into HC1 by flow reversal
2) valve is turned to diluent port and while pump P1 disposes BTB from HC1, P2 aspirates both BTB and diluent into HC2 through confluence point. Disposal and aspiration flowrates and volumes are selected to yield 1+1 mixture of BTB with diluent.
3) valve is turned toward flow cell and P2 disposes 250mcrL. This brings the central part of the mixture into the middle of the 20cm long light path.
4) the flow is stopped for 25 seconds and then resumed at a high flowrate in order to flush the system.
Response curves obtained with BTB solutions (buffered at pH 11) and with DI water as diluent (C) show a horizontal plateau, recorded during stop flow period. Absorbance values collected at the end of stop flow period (WIN) were used together with a baseline value (BS) to construct a calibration graph (D) in range 0 to 2000ppB BTB.
The slope of the thus obtained calibration line is:
0.585 mAU/1ppB BTB
measured in 20cm long light path, on a solution of BTB diluted in ratio 1 + 1. This yields the molar absorptivity of bromothymol blue
(m.w.= 625) at 620nm:
ε = 3.66x10exp4
while the value listed in the literature is:
ε = 3.57x10exp4
1.5.3.
Molar Absorptivity of Bromothymol Blue